Press Releases
JAMSTEC
Sweet coffee stains do not leave rings:
Control and mechanism of patterns left by particle-laden droplets after adding sugar
Overview
Dr. Shunsuke F. Shimobayashi from the Department of Mathematical Science and Advanced Technology of Japan Agency for Marine-Earth Science and Technology (JAMSTEC) and his colleagues have discovered that the "coffee ring effect"—the phenomenon whereby particle-laden droplets dry into ring-shaped patterns—can be controlled by adding sugar. They have also clarified the underlying mechanism.
We encounter colloidal suspensions daily, in the form of muddy water, India ink, or even a drop of coffee. When a drop of coffee gets splashed on your desk, it will dry and leave a ring-like brown stain, called the "coffee ring effect." This occurs because the evaporation rate near the outer edge of the drop is higher than at the center of the drop. As a result, the liquid flows from the droplet center to its edge, carrying coffee particles to the edge and creating a ring-shaped stain. This phenomenon can also be seen with many other particle suspensions, such as in dried traces of raindrops on a window pane.
The research group occasionally discovered that the coffee ring effect is suppressed during the evaporation of sweet coffee (Fig. 1). Using colloidal suspensions that mimic coffee drops, the authors proved that the evaporative deposit changes from a ring-like pattern to a uniform pattern through an intermediate pattern with an increase in the sugar concentration. (Fig. 2). Furthermore, by analyzing the particle behavior during drying and the internal structure of the drying deposit with confocal laser and electron microscopes, the authors revealed that sugar precipitation from the droplet edge drives the uniform stain patterns (Fig. 3, 4, 5).
It is well-known that the coffee ring effect decreases the resolution in inkjet printers and other printing devices. Hence, this discovery by Dr. Shimobayashi and his colleagues makes it possible to control the coffee ring effect in a quick, inexpensive, and harmless manner, and thus could contribute to improving printing techniques.
There are also numerous examples in nature of liquid droplets containing various substances, such as clouds, rain, and sea spray. However, the mechanism behind their formation and dissolution is still unclear because of the non- equilibrium nature. Hence, these results are expected to contribute to understanding the behaviors of such droplets.
The above study was supported by JSPS KAKENHI 18K13521.
This study was published in Scientific Reports on December 11, 2018 (JST).
https://www.nature.com/articles/s41598-018-35998-w
Title: Suppression of the coffee-ring effect by sugar-assisted depinning of contact line
Authors: Shunsuke F. Shimobayashi1, Mikiko Tsudome2, and Tomo Kurimura3
1 Department of Mathematical Science and Advanced Technology, JAMSTEC
2 Research and Development Center for Marine Biosciences, JAMSTEC
3 Institute of Innovative Research, Tokyo Institute of Technology
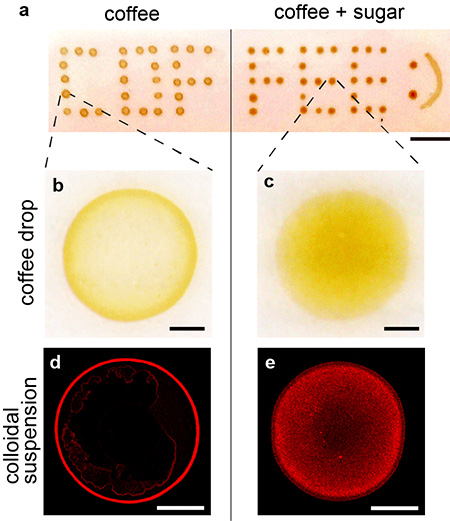
Figure 1. a) Drying patterns of coffee drops. The left half shows coffee stains without sugar; the right shows coffee stains with sugar.
b, c) Magnified part of a). A ring pattern is seen in b), on the other hand, a uniform pattern is shown in c).
d, e) Fluorescent images of the drying patterns of colloidal drops. A ring pattern is seen in d), on the other hand, a uniform pattern is shown in e). Scale bars are all 500 µm.
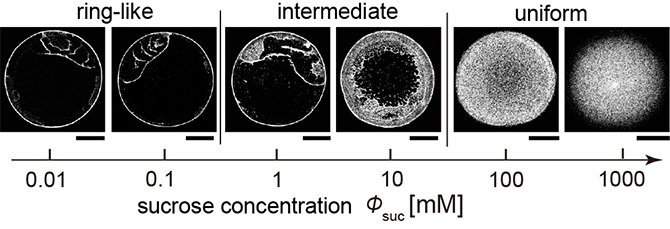
Figure 2. Deposit pattern change with the increase in sucrose concentration. The deposit patterns changes from the ring-like pattern to the uniform pattern through the intermediate pattern. The scale bars are all 500 µm.
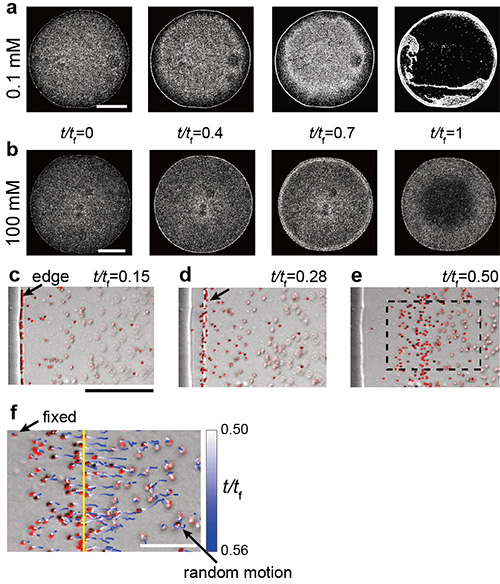
Figure 3. a)-b) Sequential images near the bottom during the evaporation of a drop. Scale bars are 500 µm. The parameter t/tf indicates the portion of time elapsed, with t/tf =1 indicating that the drop has completely dried. c-f) Particle behaviors near the droplet edge. The magnified image of a part of e) surrounded by the dashed box is shown in f). Scale bars are 50 µm for c) to e) and 20 µm for f).
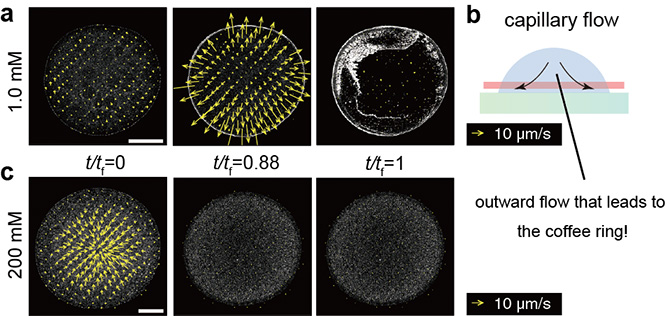
Figure 4. a) - b) Particle image velocimetry (PIV) with sugar concentration of 1.0 mM and an accompanying schematic diagram. The cross-section represented by the red line in b) is the observed plane with a confocal microscope. The yellow arrows indicate the speed of moving fluid at the site. As with standard coffee rings, particles are transported to the outer edge via an outward flow of liquid. c) PIV with sugar concentration of 200 mM. No strong outward flow can be seen, finally leading to the uniform pattern.
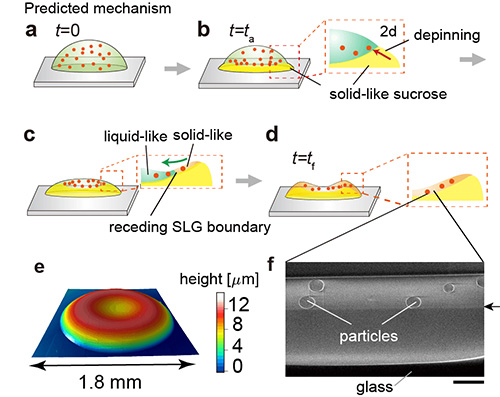
Figure 5. a-d) Predicted drying mechanism of the uniform coating.
e) Height profile of the final deposit.
f) FIB-SEM image of cross-section for the portion of d) marked by the dashed box. The scale bar is 2 µm.
Contacts:
- (For this study)
- Shunsuke F. Shimobayashi, Scientist, Department of Mathematical Science and Advanced Technology, Japan Agency for Marine-Earth Science and Technology
- (For press release)
- Tsuyoshi Noguchi, Manager, Press Division, Public Relations